Factory in Tamil Nadu Producing Toxic Cough Syrup Sealed; Director on the Run
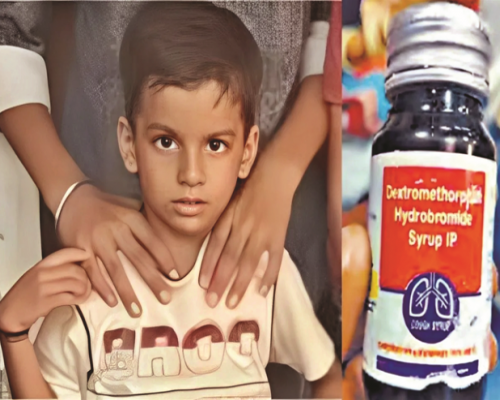
The Tamil Nadu government has sealed a pharmaceutical manufacturing unit in Cuddalore district after its cough syrup was found to be contaminated with toxic substances, allegedly causing multiple child deaths in Madhya Pradesh and Rajasthan. The company’s director is currently absconding, and police have launched a search operation in coordination with state and central authorities.
Background and Findings
According to officials, the factory—operated by Srinivasa Pharmaceuticals—was producing “Cough Syrup Coldoff”, which was distributed across several states. Laboratory tests confirmed that the syrup contained diethylene glycol (DEG) and ethylene glycol levels 486 times higher than the permissible limit set by the Indian Pharmacopoeia (IP). Both chemicals are known to be highly toxic, especially to children, and can cause kidney failure and neurological damage.
The contamination came to light after 21 child deaths were reported in Madhya Pradesh and four in Rajasthan over the past few weeks. The National Commission for Protection of Child Rights (NCPCR) and the Central Drugs Standard Control Organisation (CDSCO) initiated an immediate investigation.
Government Action and Legal Proceedings
Tamil Nadu’s Health and Family Welfare Department confirmed that the drug manufacturing license of the factory has been suspended. Officials sealed the premises and seized samples for forensic testing. Meanwhile, the company’s director, identified as Jayakumar, has reportedly gone into hiding following the registration of a criminal case under the Drugs and Cosmetics Act and sections related to negligence causing death.
State Health Minister Ma Subramanian stated that the government has issued an alert to recall all batches of the toxic syrup distributed under the Coldoff brand name. He assured that efforts are underway to locate the manufacturer and ensure accountability.
Impact in Madhya Pradesh and Rajasthan
According to reports from the Health Department of Madhya Pradesh, two more children died this week after consuming the contaminated syrup, bringing the total death toll in the state to 21. Preliminary investigations revealed that the product was supplied through a local distributor in Khandwa district, and remaining stocks have since been confiscated.
Officials in both states have directed hospitals and pharmacies to immediately cease the sale and use of the affected product. Experts have warned that DEG contamination poses a severe risk, especially to children under five years old.
National and International Concern
This incident echoes earlier tragedies involving toxic cough syrups in The Gambia and Uzbekistan, which led to international scrutiny of India’s pharmaceutical export practices. The World Health Organization (WHO) has reportedly sought updates from Indian regulators regarding the latest case.
The Tamil Nadu government has assured that strict action will be taken against all those responsible and that quality control norms in drug manufacturing will be further strengthened to prevent such occurrences.























